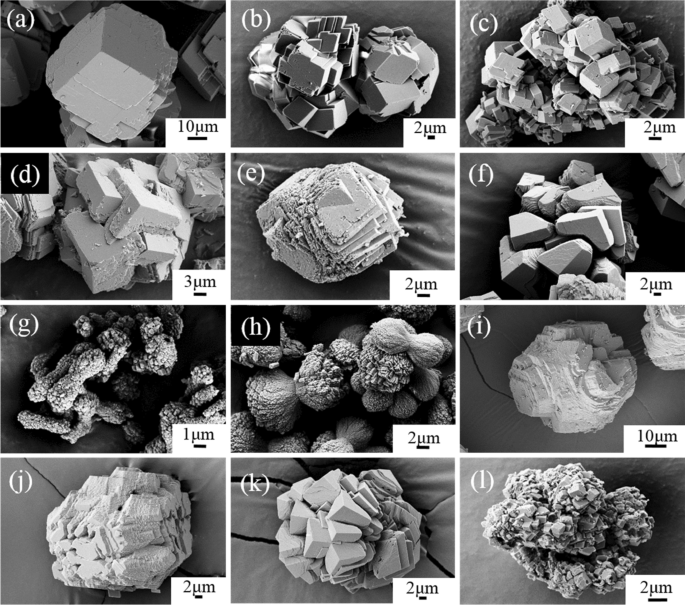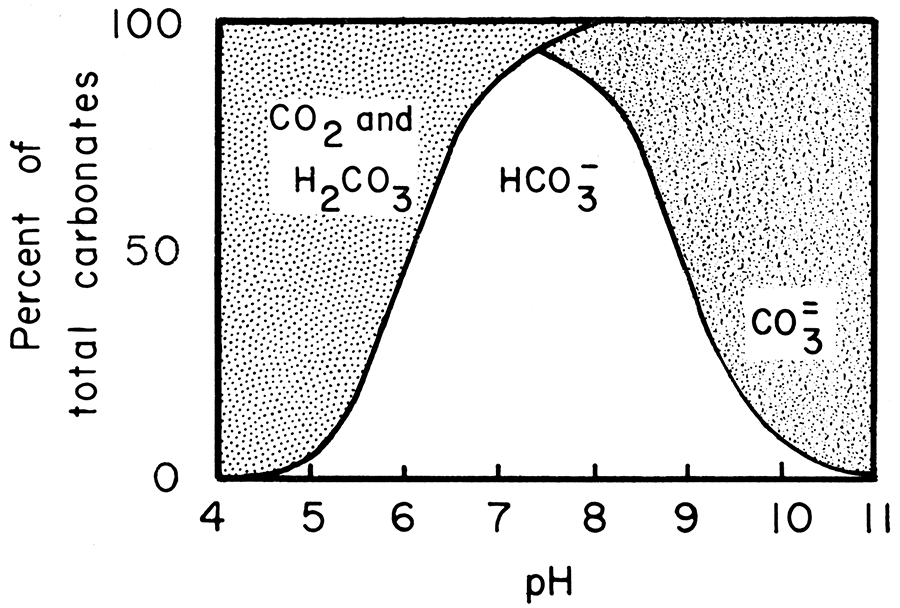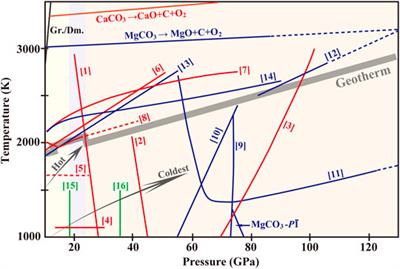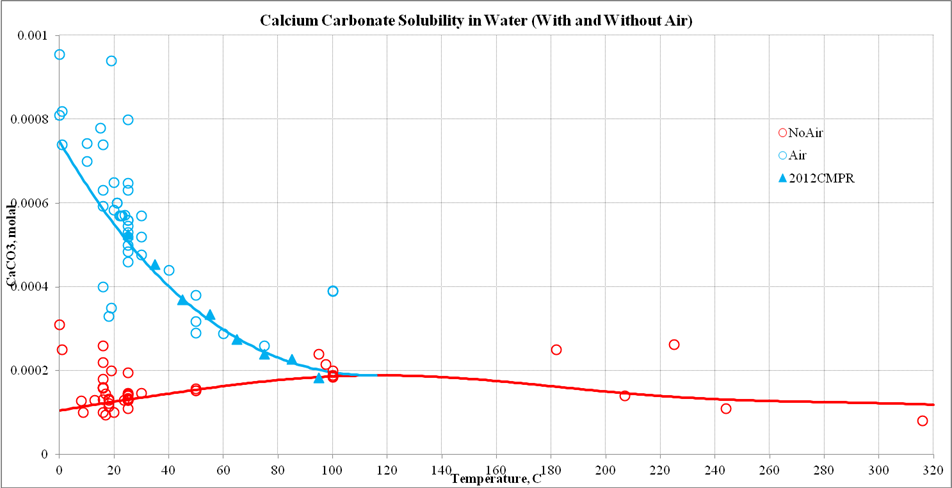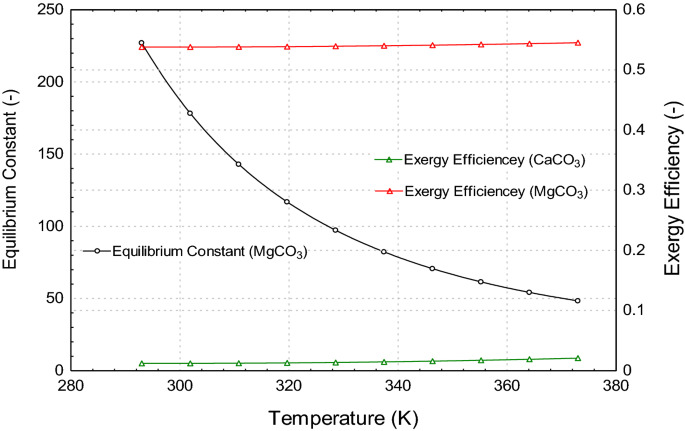
Thermodynamic analysis of theoretical dolomite formation from seawater and captured carbon dioxide | SN Applied Sciences
![The solubility (S) of dolomite [CaMg(CO 3 ) 2 ] as a function of pH... | Download Scientific Diagram The solubility (S) of dolomite [CaMg(CO 3 ) 2 ] as a function of pH... | Download Scientific Diagram](https://www.researchgate.net/publication/309875485/figure/fig1/AS:537898228826112@1505256339484/The-solubility-S-of-dolomite-CaMgCO-3-2-as-a-function-of-pH-LlogH-D.png)
The solubility (S) of dolomite [CaMg(CO 3 ) 2 ] as a function of pH... | Download Scientific Diagram
SE - Precipitation of dolomite from seawater on a Carnian coastal plain ( Dolomites, northern Italy): evidence from carbonate petrography and Sr isotopes

Low-Temperature Synthesis of Disordered Dolomite and High-Magnesium Calcite in Ethanol–Water Solutions: The Solvation Effect and Implications | ACS Omega

Solubility diagram for the system calcite/Ca-rich dolomite at T = 10°C... | Download Scientific Diagram

Solubility and Dissolution Kinetics of Dolomite in Ca–Mg–HCO3/CO3 Solutions at 25°C and 0.1 MPa Carbon Dioxide - Sherman - 2000 - Soil Science Society of America Journal - Wiley Online Library
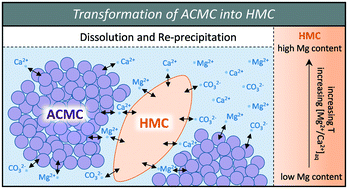
Effect of temperature on the transformation of amorphous calcium magnesium carbonate with near-dolomite stoichiometry into high Mg-calcite - CrystEngComm (RSC Publishing)

Solubility and Dissolution Kinetics of Dolomite in Ca–Mg–HCO3/CO3 Solutions at 25°C and 0.1 MPa Carbon Dioxide - Sherman - 2000 - Soil Science Society of America Journal - Wiley Online Library

Log(K) of calcite-fluid equilibrium (CaCO 3 + H + = HCO 3 − + Ca 2+ )... | Download Scientific Diagram
Difficulties are often encountered in processing heterogeneous mineral systems under conditions predicted on the basis of single

Study of Iron-Bearing Dolomite Dissolution at Various Temperatures: Evidence for the Formation of Secondary Nanocrystalline Iron-Rich Phases on the Dolomite Surface | ACS Earth and Space Chemistry

Solubility and Dissolution Kinetics of Dolomite in Ca–Mg–HCO3/CO3 Solutions at 25°C and 0.1 MPa Carbon Dioxide - Sherman - 2000 - Soil Science Society of America Journal - Wiley Online Library

Study of Iron-Bearing Dolomite Dissolution at Various Temperatures: Evidence for the Formation of Secondary Nanocrystalline Iron-Rich Phases on the Dolomite Surface | ACS Earth and Space Chemistry

Mineralogy, nucleation and growth of dolomite in the laboratory and sedimentary environment: A review - Gregg - 2015 - Sedimentology - Wiley Online Library

Calcite solubility in aqueous solution with CO2 in equilibrium obtained... | Download Scientific Diagram

Greenhouse conditions induce mineralogical changes and dolomite accumulation in coralline algae on tropical reefs | Nature Communications
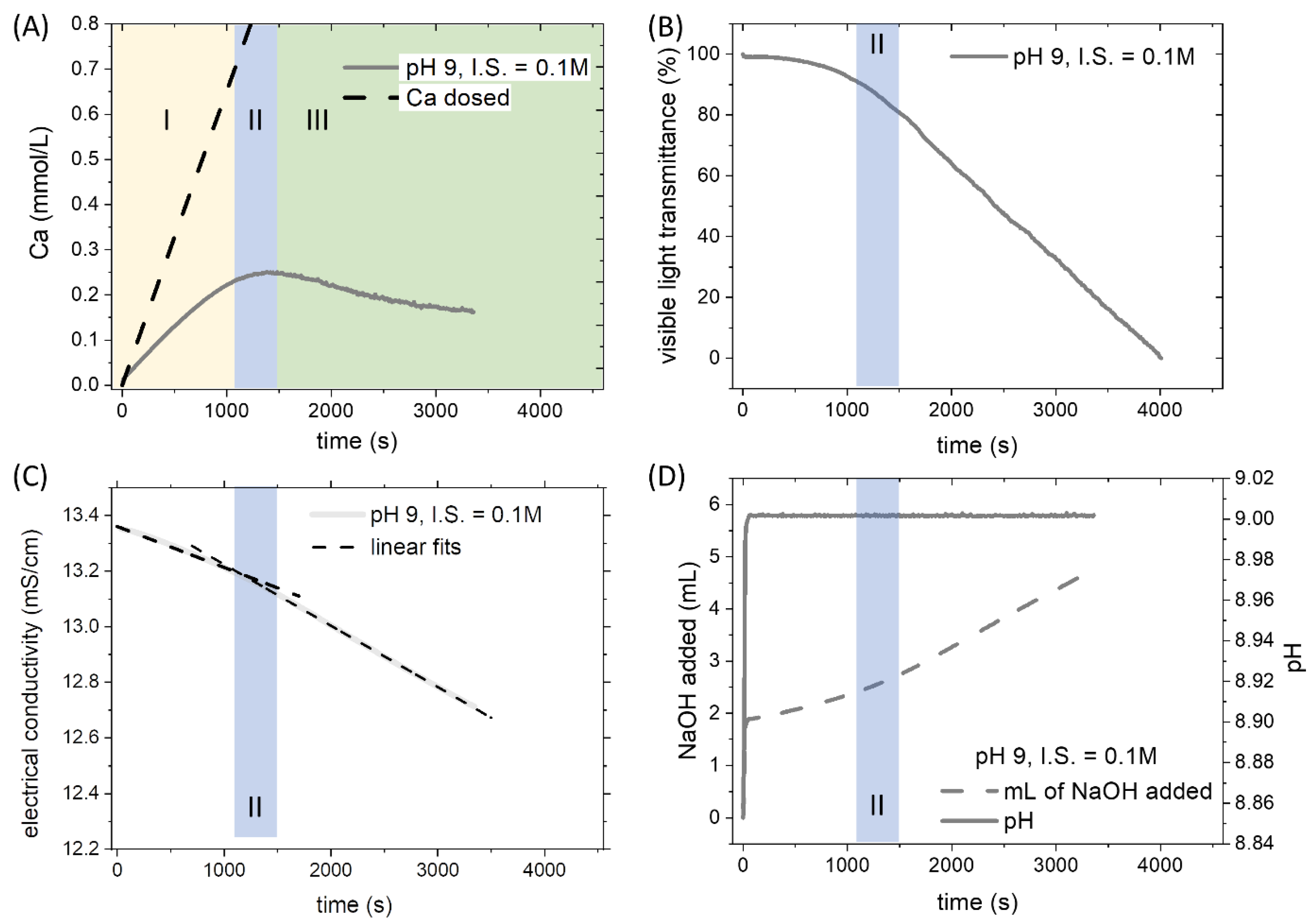
Minerals | Free Full-Text | The Effect of pH, Ionic Strength and the Presence of PbII on the Formation of Calcium Carbonate from Homogenous Alkaline Solutions at Room Temperature
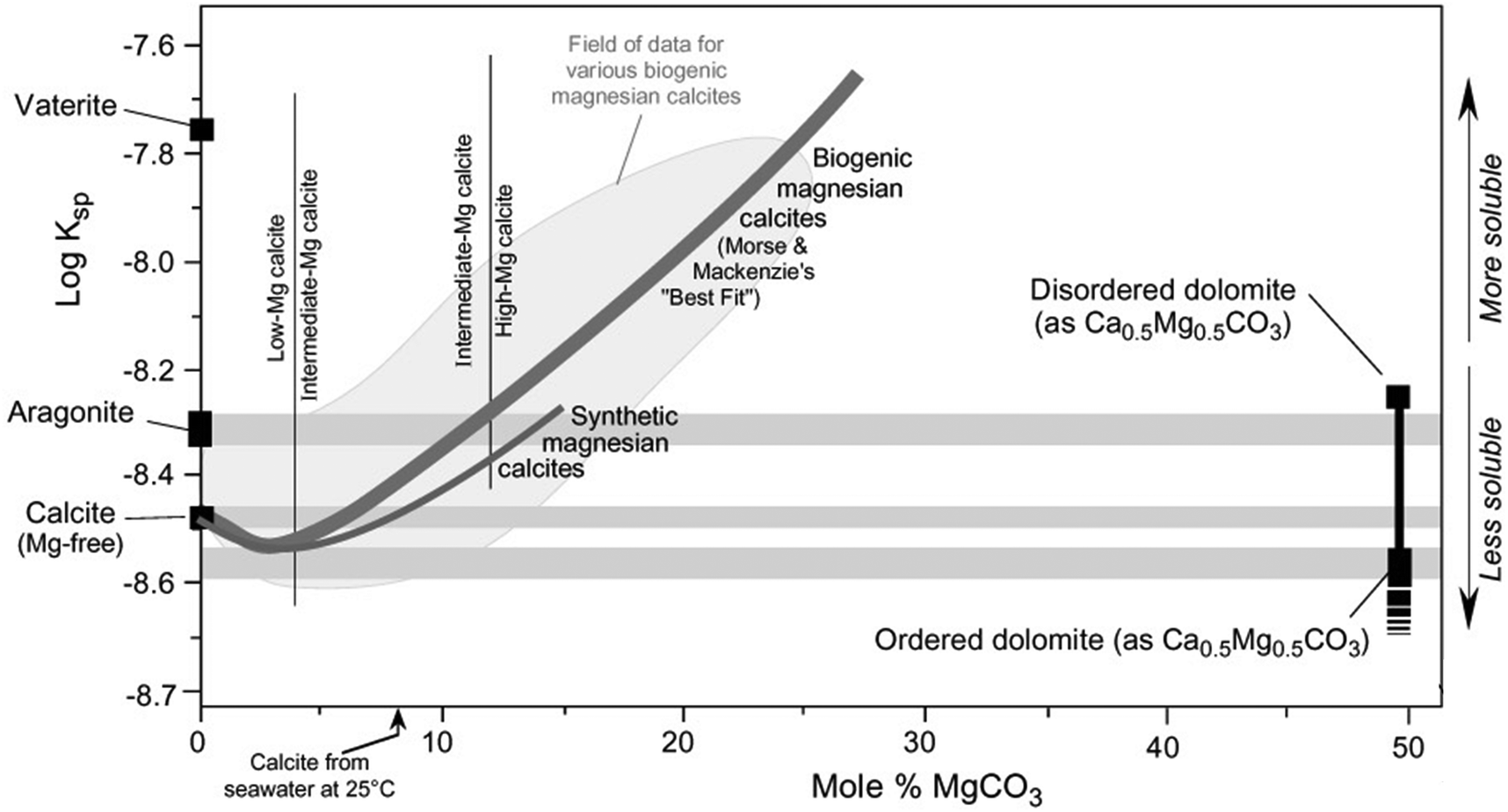
Mechanisms of Mg carbonates precipitation and implications for CO 2 capture and utilization/storage - Inorganic Chemistry Frontiers (RSC Publishing) DOI:10.1039/D2QI02482A
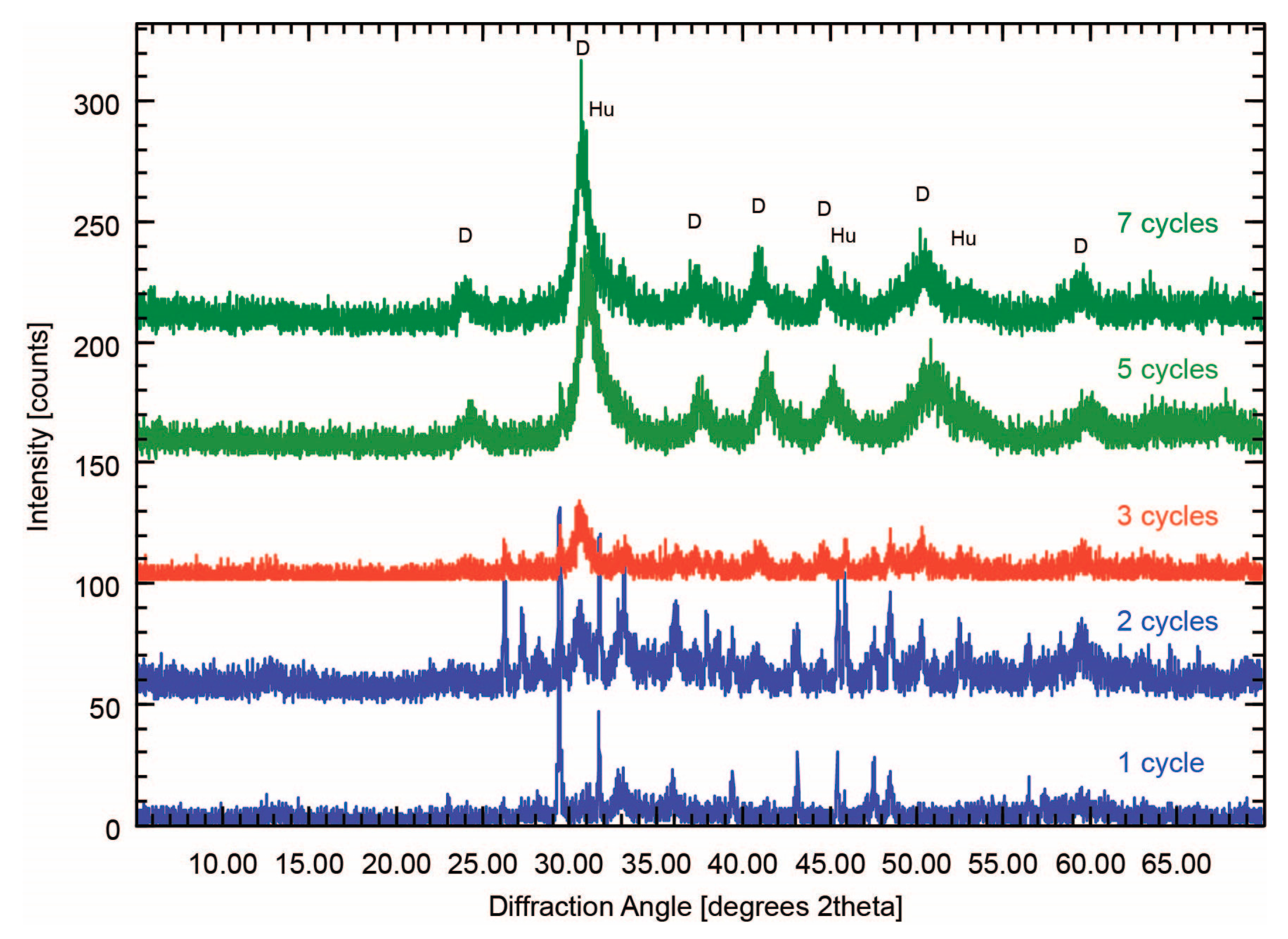
Minerals | Free Full-Text | Effect of pH Cycling and Zinc Ions on Calcium and Magnesium Carbonate Formation in Saline Fluids at Low Temperature

5 Temperature Control of Mineral Deposition – A Conceptual Overview of Surface and Near Surface Brines and Evaporite Minerals